[ad_1]
SUMMARY BOX
-
Public-health voluntary licensing of intellectual property rights has been highly effective in supporting the scale up of WHO-recommended HIV and hepatitis C virus treatments in low-income and middle-income countries, saving both money and lives.
-
Despite the success and establishment of public health voluntary licensing through the Medicines Patent Pool (MPP) as the gold standard for large-scale access to medicines, there are recurring demands for these licences to be even better, for example, encompassing greater geographic territories.
-
Observers have also raised concerns that voluntary licences may entrench intellectual property rights, be used as a mechanism to manage competition, collect royalties and segment markets (instead of supporting access) in the absence of patent rights.
-
MPP licences are generally more access friendly and include less restrictive terms than licences negotiated bilaterally between patent holders and generic manufacturers.
-
They are also publicly available, which has helped establish global norms for public health licensing.
-
Evidence suggests that MPP public health licences lead to stronger sustainable generic manufacturer competition in more countries faster, driving medicine prices down to lower than tiered prices, creating both economic and health impact.
-
This Analysis discusses the case for public-health voluntary licensing; the considerations for balancing key public health needs; the challenges, negotiation complexities and compromises at play; and the processes for assessing the value of and externally validating proposed licensing agreements in the context of a strategic, agile and voluntary mechanism.
-
Contextualising MPP’s work from a perspective of internal understanding of the intricacies of the mechanism, in light of its strengths and limitations, managing expectations, identifying where the model may provide the most added value, and building the necessary support for its sustainable application may help calibrate expectations, develop incentives to expand applications and overall reinforce the approach.
-
Public-health licensing has an important role to play in improving access to medicines, although it remains underused; this is particularly relevant to ongoing discussions that are taking place at the WHO, Intergovernmental Negotiating Body and G20, to name a few.
Introduction
In the context of access to medical countermeasures for pandemic preparedness and response, many discussions have focused on intellectual property (IP) and its role as a driver of innovation, but also its potential to delay access if not managed through a public health perspective.1 The recent prolonged discussions at the World Trade Organisation around an IP waiver under the TRIPS agreement show that there remains considerable disagreement about how to balance innovation and equitable access.2 3 One way that has been tried and tested to enable expanded access to innovative medicines across low-income and middle-income countries (LMICs) is public health oriented non-exclusive voluntary licensing of IP.4 This approach has been shown to lead to stronger sustainable generic manufacturer competition in more countries faster, driving medicine prices down to lower than tiered prices, and creating both economic and health impact.5–12
In the 2000s, one of the most pressing concerns for global public health was the lack of access to affordable life-saving drugs for the treatment of HIV in LMICs. The newer, more effective and better tolerated antiretrovirals, some of which were needed for second line treatment, were patented and expensive. To address this, Unitaid set up the Medicines Patent Pool (MPP) in 2010.13–15 The concept proposed was for MPP to persuade patent holding pharmaceutical companies to grant licences covering their medicines’ IP to MPP. MPP would then sublicense these to multiple generic manufacturers, thereby enabling competition around the manufacturing of affordable, quality-assured versions of those drugs for use in LMICs, especially in countries where the HIV burden was highest. In parallel, patent holding companies would continue selling their own branded products in their main commercial markets. By the end of 2021, people living in 148 countries had received close to 27 billion doses of WHO recommended HIV and hepatitis C virus (HCV) medicines through MPP-licensed generic manufacturers at remarkably low prices.16 As an example, the price of WHO-recommended daily first-line fixed-dose combination HIV treatment (tenofovir/lamivudine/dolutegravir) is now less than US$50 per person per year: less than US$1 per week.17
In light of this success, MPP has progressively expanded its mandate from HIV medicines, to medicines for hepatitis C and tuberculosis, followed by a broader expansion into medicines either on the WHO Model List of Essential Medicines or with strong potential for future inclusion, and since 2020, COVID-19 health technologies, long-acting technologies and drug formulations, and biotherapeutics (such as monoclonal antibodies).4 5 18 19 The MPP network of generic manufacturers now encompasses more than 50 companies in 16 countries across all continents.20 Through HIV and HCV treatments alone, the estimated impact of this work should reach US$3.5 billion saved and 160 000 deaths averted by 2030.20
A recent independent Lancet viewpoint suggested this model should be further promoted and incentivised, including in contexts of pandemics.21 However, beyond MPP’s impact, there is limited understanding of the intricacies of the model, the modalities by which it works in practice, the levers involved and the trade-offs made. Such knowledge may be critical in framing the value of public-health voluntary licensing in ongoing discussions on pandemic preparedness and equitable access taking place at the WHO, Intergovernmental Negotiating Body and G20. This article presents an inside perspective on negotiating public-health voluntary IP licensing agreements to increase access to health technologies. Other mechanisms to facilitate access to medicines exist, some of which also focus on IP, and are discussed elsewhere.8 9 22–27
The case for public-health voluntary licensing
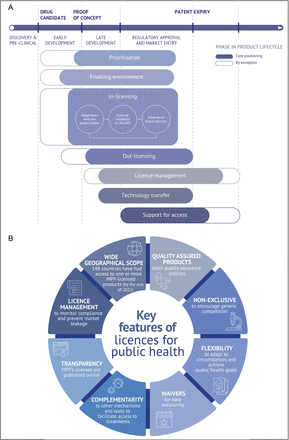
Before approaching a patent holder to discuss in-licensing of a patented medicine, MPP does a careful assessment of the public health need for the medicine in question, analysing the burden, needs of affected communities, clinical data, normative guidance, regulatory pathway and other information, in addition to holding conversations with clinical experts, government and United Nations officials, and affected community groups.28 The case for licensing may then include elements like disease burden, unmet need/demand, equity concerns, public opinion (both positive and negative), geographic reach, licence management (which MPP undertakes pro bono), cost/benefit analysis and, increasingly, investor preferences, including intersections with environmental, social and governance (ESG) frameworks. It can sometimes take several years to persuade a patent holder to give MPP a licence—and in-licensing is only one part of the voluntary licensing life cycle (figure 1A).
The context and key features of voluntary licensing for public health. (A) Complementary voluntary licensing areas of work and the medicine development to access life cycle. While this paper focuses on in-licensing as the central and most scrutinised element of MPP’s voluntary licensing work, there are other critical areas of contribution for MPP both upstream (ie, identification of suitable candidate medicines through prioritisation and development of an enabling environment for in-licensing) and downstream (ie, out-licensing, licence management, technology transfer and support for access). As mentioned in MPP’s strategy for 2023–2025, MPP in-licensing activities have generally started around late product development, regulatory approval and early market entry.28 In some cases, in-licensing efforts have begun after an approved product was prioritised by a global health mechanism (such as the WHO Model List of Essential Medicines). Moving forward, MPP in-licensing efforts will take place more upstream to embed access considerations earlier in the innovation process, support product development, and shorten the time from product approval to affordable access in LMICs. MPP will also increase its work further downstream to support affordable access to licensed products and, in exceptional circumstances, continue to work on licensed products beyond patent expiry if that can help pave the way for future priority products. (B) Key features of voluntary licensing agreements aimed at maximising the public health impact of priority products. Insights on how these features have been implemented across different licences are available in online supplemental appendix. EAG, Expert Advisory Group; MPP, Medicines Patent Pool.
There are a number of reasons why patent holders may want to consider public health voluntary licensing and this can impact the length and complexity of negotiations. Those four main reasons are:
-
Improving reputation. Access to medicine advocates, affected communities and even governments have been very active, publicly calling out companies that fail to ensure broad access to life-saving treatments.29 Recently highlighted during the COVID-19 pandemic, vaccine inequity has also dramatically increased the focus on equitable access.30 Originator companies lacking an access programme for critical health products may be exposed to widespread public criticism, while having a good access programme may conversely attract praise.31 32 The Access To Medicine Index (ATMI) provides a formal tool to assess some of the largest pharmaceutical companies according to the quality of their LMIC access programmes. ATMI ratings, which attract increasing attention, consider MPP licences as gold standards for large-scale access to medicines, offering ‘advantages from a global health perspective’, including ‘access-friendly terms’.31
-
Operational capacities. Even where originator companies genuinely want their products to make the biggest impact, they often have little presence or experience in many LMICs. Moreover, companies’ ability to engage with public stakeholders to support scale-up and overcome access barriers may be limited, partly due to frameworks aimed at mitigating potential conflicts of interest, while MPP, as a not-for-profit independent public-health organisation founded by Unitaid, is ideally placed to work with global health stakeholders and support access across LMICs.33 34
-
Meeting large-scale demand. Some originator companies may have limited capacity for manufacturing a given product at the scale needed. And there may be little interest for them to invest into increased capacity to match the demand of what may be considered low-margin markets outside of their commercial priorities. As such, licensing to generic companies geared towards maximising economies of scale is a mutually beneficial compromise.28
-
Financial efficiency and returns. There are significant costs associated to registration and distribution of a product in each individual country, and MPP-licensed generic companies usually cover those. Furthermore, even in the case of bilateral agreements between an originator and generic companies, the originator must commit human and financial resources to manage a licence, whereas MPP undertakes this pro bono as a standard part of its work. MPP licences can also include royalties, which can be significant. Moreover, as investors in originator companies become increasingly engaged with ESG issues, there can be a tangible benefit in having a good—early, broad, effective—access programme and a substantial cost in failing to have one. For example, 133 major asset managers, collectively handling more than US$21 trillion, have signed up to use the ATMI in their investment decisions, and some pharmaceutical companies have issued bonds that cost them lower interest rates when access is ensured across LMICs.32 35 36 Finally, voluntary licensing to generic manufacturers can help overcome the challenge of external reference pricing that pharmaceutical companies face for their commercial markets in higher-income countries when exploring discounted pricing options for LMICs.37 38
Striking a balance to meet public health needs
One major feature of the MPP model is that it requires carefully balancing the often-different needs of innovators (patent holders), generic companies and other global health stakeholders, including affected communities, civil society organisations, international funding and procurement agencies, and national governments. Those stakeholder needs, and their effects on expressed preferences and concerns when it comes to IP management and access, can vary across time depending on the environment, disease and medicine. The model should be win-win-win for all stakeholders directly and indirectly involved. However, competing interests unsurprisingly arise and concessions from all parties are often required. In a 2020 report, the Médecins Sans Frontières (MSF) Access Campaign highlighted 11 key issues and concerns with regards to the use of voluntary licensing to expand access to medicines in LMICs, most notably: the lack of transparency; geographic scope limitations; restrictions on the source and production of active pharmaceutical ingredients; and antidiversion requirements.26 Public health being of paramount importance, MPP strives to adhere to certain key elements in its licences (figure 1B), some of which address challenges and opportunities highlighted by MSF and other observers.26 27 31 For example, MPP licences are transparent (published on MPP’s website) and are generally more access friendly and include less restrictive terms than licences negotiated bilaterally between patent holders and generic manufacturers, without the contribution of MPP as a broker.21
The essential parts of MPP licences cover: the territory where the generic product can be manufactured and/or sold; what the licensees can do (eg, which active ingredient or formulations they can manufacture and sell); what the product can be used for; quality requirements; compliance monitoring and sublicensee management aspects, including sales reporting; royalties, if applicable; technology transfer, when required; and compatibility and complementarity with other access mechanisms. Each agreement is bespoke, meaning there is no standard agreement, although MPP has created precedents around some key terms that are habitually adhered to (and which have in some cases helped improve pre-existing bilateral agreements, thereby addressing some of the challenges highlighted by access to medicine advocates).26 MPP’s commitment to transparency by putting its licences into the public domain has been instrumental in creating these precedents and, more generally, in establishing global norms for public health licensing (based on the most effective licence clauses, as per MPP’s experience and learnings).39
The territory covered by the licence, which is what often attracts most scrutiny, needs to be sufficiently large to make a significant positive impact on public health and be financially attractive to generic companies, ensuring a sustainable market with economies of scale to drive prices down. This must be balanced against the originators’ commercial aspirations, not just for the product under discussion but for their overall portfolio (eg, broad considerations around establishing precedents, or country-specific considerations in light of companies’ in-country presence in certain markets). Some observers have noted that MPP licences often do not cover many upper-middle-income countries (UMICs), including some with high disease burden.26 While this has often been true for certain large UMICs where pharmaceutical companies have substantial commercial interests, it must also be said that many smaller and mid-size UMICs have also benefitted from MPP licences, most of which would not have had access to innovative products in generic versions until patent expiry.40 For example, approximately 50% of both health and economic impact of MPP licences for DTG-based HIV treatment regimens has been shown to arise from access in UMICs (34 by the end of 2022).5
In the context of efforts to maximise the allowed territory, MPP has used segmentation around target populations (eg, this has been used to allow a larger territory for paediatric use—see online supplemental appendix) and markets (eg, this has been used to include the public market for some countries that would otherwise have been excluded given commercial interests of the patent holder in the private market—see online supplemental appendix). Low-level royalties have also been used to broaden allowed territories (including for coverage of additional UMICs), as discussed later.41 And some clauses (discussed later and in online supplemental appendix) have also enabled, under certain conditions, supply outside the nominal territory of countries listed in a licensing agreement. As is possible for other clauses, improvements regarding the licensed territory can be—and sometimes are—pursued following initial signature of the licensing agreement (ie, efforts can be made to expand the covered territory, as has happened for many, but not all, licences—see online supplemental appendix). However, expansions of the licence territory are challenging, as they often relate to countries where the innovator may have commercial interests and may sometimes not materialise despite MPP’s efforts and, at times, advocacy from other stakeholders.42
Beyond access to the finished products, some licences have allowed manufacturing but not sales in certain countries. These are countries with significant manufacturing capacity (eg, China and South Korea) where the patent holder intends to supply the innovator product, but where it agrees to allow manufacturing as a way to support more diverse and secure supply capacity for other markets, the importance of which the COVID-19 crisis highlighted. Unfortunately, while the focus of this approach may be on the benefit it brings, it is also recognised that this at times can create challenges for manufacturers vis-à-vis their local populations.42
Quality assurance (eg, by stringent regulatory authorities, SRA) is essential and details of how this is achieved are often the subject of discussion with originators and generic companies. For COVID-19 products there has been a need to balance speed to market against strict quality assurance. For example, one of MPP’s recent licences, rather than requiring the product to achieve SRA approval or WHO Prequalification (PQ) before sales could take place, as is usual, waived that requirement and only required application for PQ, provided the product was approved in the countries of manufacture and sale, and that PQ was subsequently achieved within 12 months.43 While the fastest HIV and HCV MPP-licensed generic development-to-market timelines had taken 3–4 years, the COVID-19 crisis showed us that it is possible to accelerate timelines substantially, with MPP-licensed COVID-19 antivirals molnupiravir and nirmatrelvir being developed in less than a year following the signature of licences (which were negotiated in under 6 months, which is also faster than most negotiations for previously licensed MPP products).44–46 A more detailed analysis of MPP’s experience during the COVID-19 pandemic will be presented elsewhere.
MPP licences generally bear no or limited royalties payable by generic companies to originators, and MPP has generally avoided royalties on paediatric products, for low-income countries, and on countermeasures for a Public Health Emergency of International Concern. However, to broaden the scope of a licence to include additional markets (eg, private markets) or countries which would otherwise be excluded by an originator wanting a commercial return, MPP has been willing to negotiate royalties that could be tiered (eg, according to country per capita income levels). For example, one MPP licence for four UMICs includes royalties which are commercially interesting for the originator and enable a sufficiently low price such that the governments involved can afford to significantly scale up access.41 Royalties can also be intended for reinvestment into public health initiatives.47
At times, advocates have raised concerns that voluntary licences may entrench IP rights and be used as a mechanism to manage competition or segment markets (rather than to support access) and to collect royalties in the absence of sufficient patent rights to ensure exclusivity. This is why MPP has insisted on a key principle: that licences should not create contractual barriers where no IP rights exist.27 Indeed, no country should be worse off because of an MPP licence that should, at the same time, improve access in many countries. As part of MPP’s public health approach, licences must also be compatible with the application of other access to medicine mechanisms, such as TRIPS flexibilities.48 As a result, MPP has pioneered the following concepts, and through the publication of its licences has supported their broad adoption:
-
Commitments on the part of originators to waive data exclusivity for relevant markets, as such exclusivity can prevent generic companies from selling in a country.
-
Where there are royalties payable, an agreement that these are only due in countries with relevant patents.
-
The ability for sublicensees to sell outside a licensed territory in circumstances in which there is no patent infringement, including in the case where TRIPS flexibilities have been used for the granting of a compulsory licence, a patent opposition has been validated, or a patent has been rejected following thorough examination or withdrawn by the patent holder or applicant.49 This means that the effective territory for some licences may in fact end up being much larger than the nominal territory set out in the licence.50
-
The ability of sublicensees to terminate agreements unilaterally when circumstances change, such as when they no longer need a licence because remaining patents are not considered to be blocking.
Additional details on various ways that the key features of MPP licences discussed above are implemented (in addition to other elements, such as how licences have included the provision of technology transfer documentation) are available as online supplemental appendix.
The negotiation process and some of its complexities
Perhaps not surprisingly, negotiating these elements can be complex, especially since negotiations cover not only the licence between the originator company and MPP (the head licence) but also the sublicence that MPP will sign with generic companies, which will be the same across all sublicensees, owing to the necessity of treating sublicensees equally. Negotiations can be complicated by differences of opinion within originator companies (eg, between the legal team, which is often very cautious, and the commercial and access teams, which may see access from different perspectives). Historically, negotiating MPP licences has taken anywhere from 6 weeks to 18 months; and some negotiations have been dropped or have failed.
This is important to keep in mind as MPP has limited leverage per se, and levers are primarily external, for example, coming from treatment advocates, governments, investors, media, public opinion and other stakeholders who might ask for access to specific products by direct or indirect interactions with patent holding companies developing and marketing those medicines. In some cases, country requests or public discussions on how to improve affordability of a given product in a given country may have played a role in tipping a company’s interest towards voluntary licensing as a proactive way to address the issue.
While licence agreements themselves are fully public (with one exception), negotiations are almost always covered by a confidentiality agreement, and it is therefore not possible to inform stakeholders in real time on progress. However, MPP seeks inputs from various stakeholders and takes those into consideration during talks; and feedback is obtained from members of MPP’s Expert Advisory Group (EAG), Scientific Advisory Panel (SAP) and the new Community Advisory Panel (CAP), but also more broadly from partners across various organisations working in the global health space, including at times governments, which in some occasions have specifically requested the negotiation of a given licence.51 52
Sometimes MPP has walked away if satisfactory terms could not be agreed. As such situations are covered by confidentiality agreements, no one typically knows that MPP has walked away and certainly no one knows the reasons. And there are occasions in which negotiations may stop, stall, or pause, and later reopen, with the potential to eventually lead to a successful agreement. But, again, details of those negotiations are rarely public knowledge, even if the final agreement is public. Finally, and very importantly, some patent holders do not want to engage with MPP at all, or are ready to do so in relation to certain (relatively small) territories, or only for some indications or disease areas.
External validation of proposed licensing agreements
Once MPP management is convinced that it has achieved the best licence possible under the circumstances, and that moving forward would make a significant improvement to public health (or could have strategic significance leading to a change in paradigm—as did the first MPP licence, with the US National Institute of Health, that contributed to giving credibility to MPP as a new mechanism), the potential licence is sent for assessment by MPP’s independent EAG, which is supported by members of the SAP and CAP.51–53 The EAG is asked two questions:
-
Does the licence offer sufficient added value over the status quo?
-
Does the licence sufficiently meet the requirements of MPP Statutes (eg, quality requirements, compatibility with TRIPS flexibilities, anti-diversion mechanisms as essential measures to ensure that licences are implemented properly, which is key for MPP to be able to conclude additional licences in the future)?54
The EAG reviews the proposed licence and captures its assessment in a report to the MPP Governance Board, recommending for or against the licence. If the Governance Board approves the licence and the Executive Director signs it, the report of the EAG becomes a public document too.39 But the process does not necessarily end there, and MPP has had the ability to improve several licences subsequently through amendments regarding the covered territory or other terms and conditions (online supplemental appendix).
Conclusion
MPP is a unique organisation that has built distinctive expertise and experience in public-health licensing of IP. The model is not perfect—no access mechanism is.8 9 22–26 However, its impact to date suggests it has an important role to play in conjunction with other access to medicines mechanisms and that, in many disease areas, it remains underused, to the detriment of people in LMICs needing access to key health technologies as urgently as anyone in high-income countries.5 18 19 Understanding some of the complexities of its model may be useful in calibrating expectations and developing incentives and supportive policies to expand its applications, in addition to MPP learning from past experiences, in terms of understanding which provisions can be most impactful and how to best cater for public health needs in the context of complex negotiations.21 26 40 This paper focuses on in-licensing aspects. However, in-licensing is only part of the process, and the work of many partners (including MPP for sub-licensing to, and managing, generic manufacturers) is needed for implementation of a licence to result in affordable access to medicines on the ground, as a licence on its own does not lead to access.
As part of its new strategy for 2023–2025, MPP will continue addressing the access challenges made highly visible during the COVID-19 pandemic.28 Beyond infectious diseases, where MPP has had most of its experience, the access model will be applied to non-communicable diseases, for small molecules—including more complex formulation technology applications—and biologics. MPP will also expand its contribution to a more diversified and sustainable manufacturing capacity, with new efforts focused on local/regional production and technology transfer, particularly for more complex products.18 19
More than 20 years into the 21st century, it seems unconscionable that where one lives can still determine whether one has access to healthcare and therefore lives or is denied a healthy life. COVID-19 has placed the public health spotlight on equity and access, and, with other efforts to facilitate uptake of essential medicines, public-health voluntary licensing has a role in addressing ongoing access to medicine inequity.55 56
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Acknowledgments
The authors thank Amina Larbi, Chan Park, Esteban Burrone, Gelise McCullough, Lobna Gaayeb,
Nataliya Omelchuk, Sophie Thievenaz and Valentina Ndibalema for their support informing the review and finalisation of this paper. The authors also thank members of MPP’s Governance Board, Expert Advisory Group, Community Advisory Panel and Scientific Advisory Panel, as well as MPP staff and many partners, including patent holders, generic manufacturers, procurement agencies, funders, governments, civil society and the various communities of people affected by diseases across low-income and middle-income countries. Finally, the authors also acknowledge Unitaid, the Swiss Agency for Development and Cooperation, the French Ministry for Europe and Foreign Affairs, the Ministry of Foreign Affairs of Japan, the German Agency for International Cooperation, and the Wellcome Trust for their past and/or ongoing financial support of MPP’s work on voluntary licensing of intellectual property. Funders did not have a role in the development of this paper.
[ad_2]
Source link